
One of the model species for studying microevolutionary issues is a small insectivorous mammal - the common shrew Sorex araneus Linnaeus 1758, which lives in Northern Eurasia from the British Isles to Yakutia. This species is notable primarily for its unusual sex determination system, namely the presence of a sexual trivalent in males (XY1Y2). Moreover, among all shrews, it is S. araneus that is characterized by amazing karyotype variability - at least 76 intraspecific chromosomal races, differing in the structure of karyotypes, have been described to date. The races are distributed parapatrically and, at areas of contact, form hybrid zones, the width of which is inversely proportional to the degree of differences in karyotypes. Of the 36 hybrid zones studied, the most interesting was the zone between the “Moscow” and “Seliger” races on Valdai, since these races are characterized by maximum karyotypic differences and in meiosis, natural F1 hybrids form the longest currently known configuration - a multivalent of 11 chromosomes (CXI).
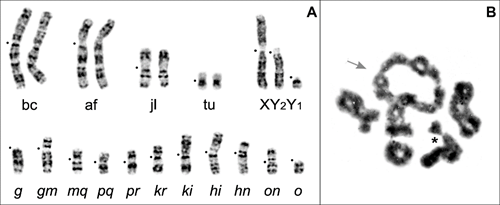
It was assumed that such multivalents could disrupt the course of meiosis, and as a result, hybrids would either suffer from reduced fertility or be completely sterile. And, therefore, gene flow in such hybrid zones should be sharply limited.
A team of authors from the Institute of Ecology and Evolution of the Russian Academy of Sciences (S.V. Pavlova and N.A. Shchipanov) and IOGEN RAS (S.N. Matveevsky and O.L. Kolomiets) for the first time used an expanded set of modern cytogenetic and immunocytochemical methods, and also used electron microscopy for analysis of karyotypes and the course of meiotic divisions in pachytene spermatocytes in natural maximally karyotypically “complex” F1 hybrid males between the “Moscow” and “Seliger” races, carrying the CXI-multivalent.
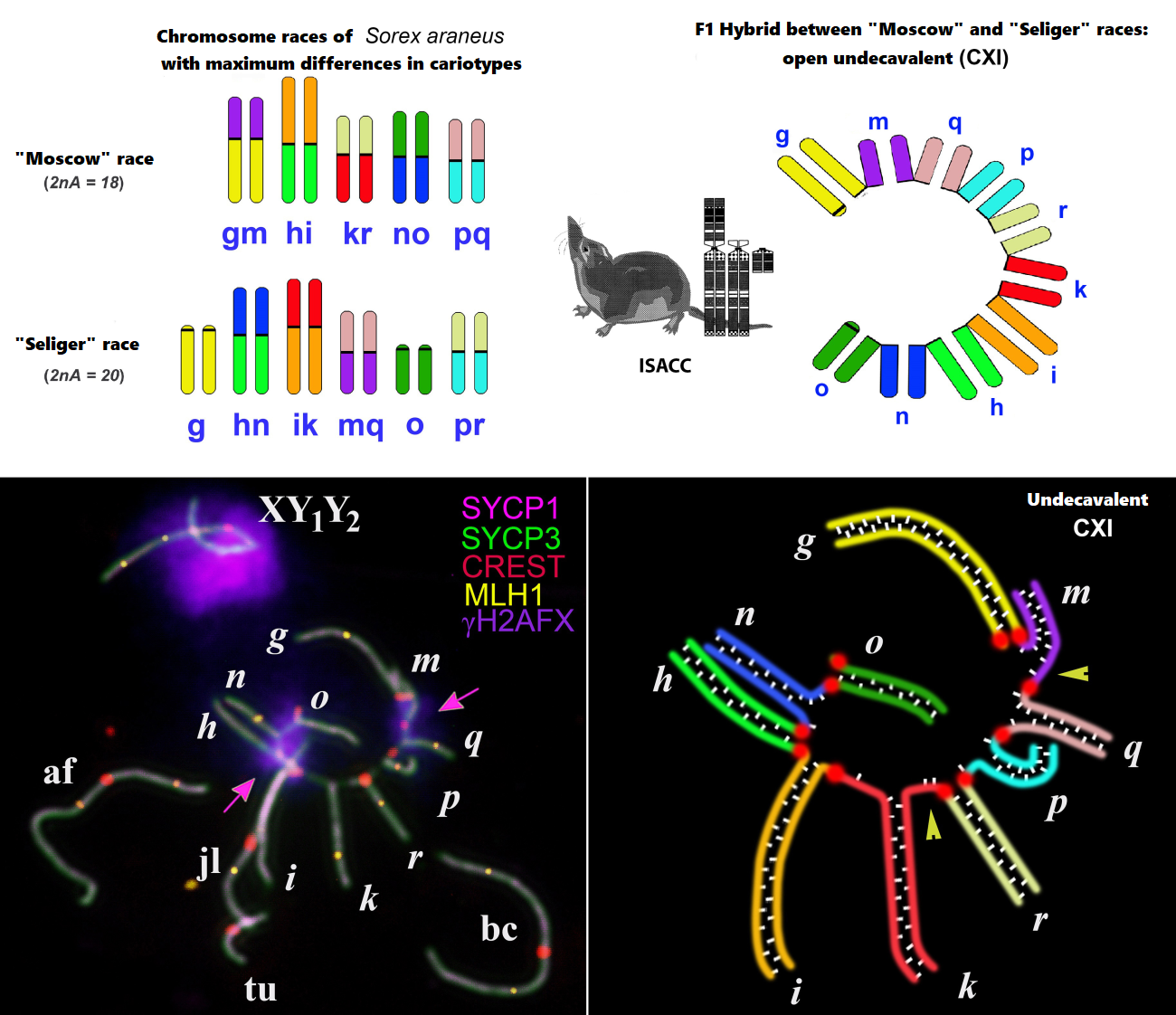
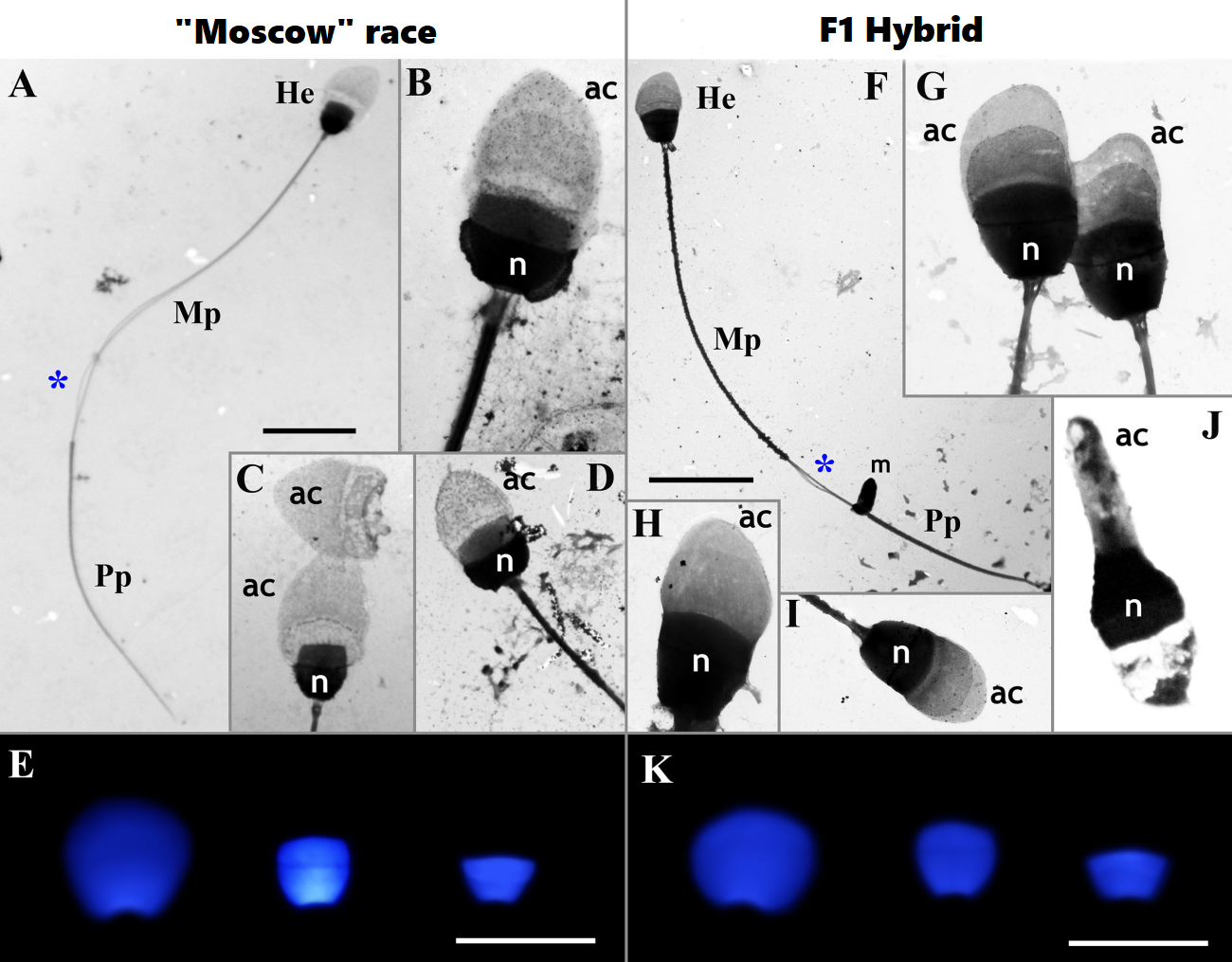
It turned out that despite the minimal proportion of anomalies found in spermatocytes, such as chromosome associations, stretched centromeres and the absence of recombination nodes in some chromosomal arms of the CXI multivalent, F1 hybrids have a large number of morphologically normal active sperm. All other studied meiotic patterns also did not differ significantly from those in individuals of “pure” races.
Thus, the study showed that the carriage of a whole set of structural chromosomal rearrangements does not lead to fatal sterility of F1 hybrid males, and therefore, the probability of free gene flow between parapatric chromosomal races of common shrews should be quite high.
However, the paradox is that the hybrid zone between the “Moscow” and “Seliger” races is one of the narrowest, only 1-2 km wide, and therefore the question of the mechanisms for maintaining the stability of the areas of hybridizing races remains open.
The work was carried out with financial support from the Russian Science Foundation (grant № 22-24-00285, https://rscf.ru/project/22-24-00285/)
(Q1) Matveevsky S.N., Kolomiets O.L., Shchipanov N.A., Pavlova S.V. 2024. Natural male hybrid common shrews with a very long chromosomal multivalent at meiosis appear not to be completely sterile // Journal of Experimental Zoology Part B: Molecular and Developmental Evolution, 342: 45–58. https://doi.org/10.1002/jez.b.23232
